Why Does Salt Slow Down Melting Of Ice . So, more ice melts than forms. Melting ice absorbs energy, lowering the temperature. Salt lowers the temperature of ice water. The melting process is at the. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. The more surface area salt can cover, the better the chances for melting ice. it is well known that when you add salt to ice, the ice not only melts but will actually get colder. when salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same. why does salt melt ice. salt makes ice water colder by lowering the temperature at which water freezes. Salt only helps if there is a little bit of liquid water available. The salt has to dissolve into its ions in order to work. When you mix salt onto that layer, it slowly lowers its melting point. This phenomenon is called freezing point depression.
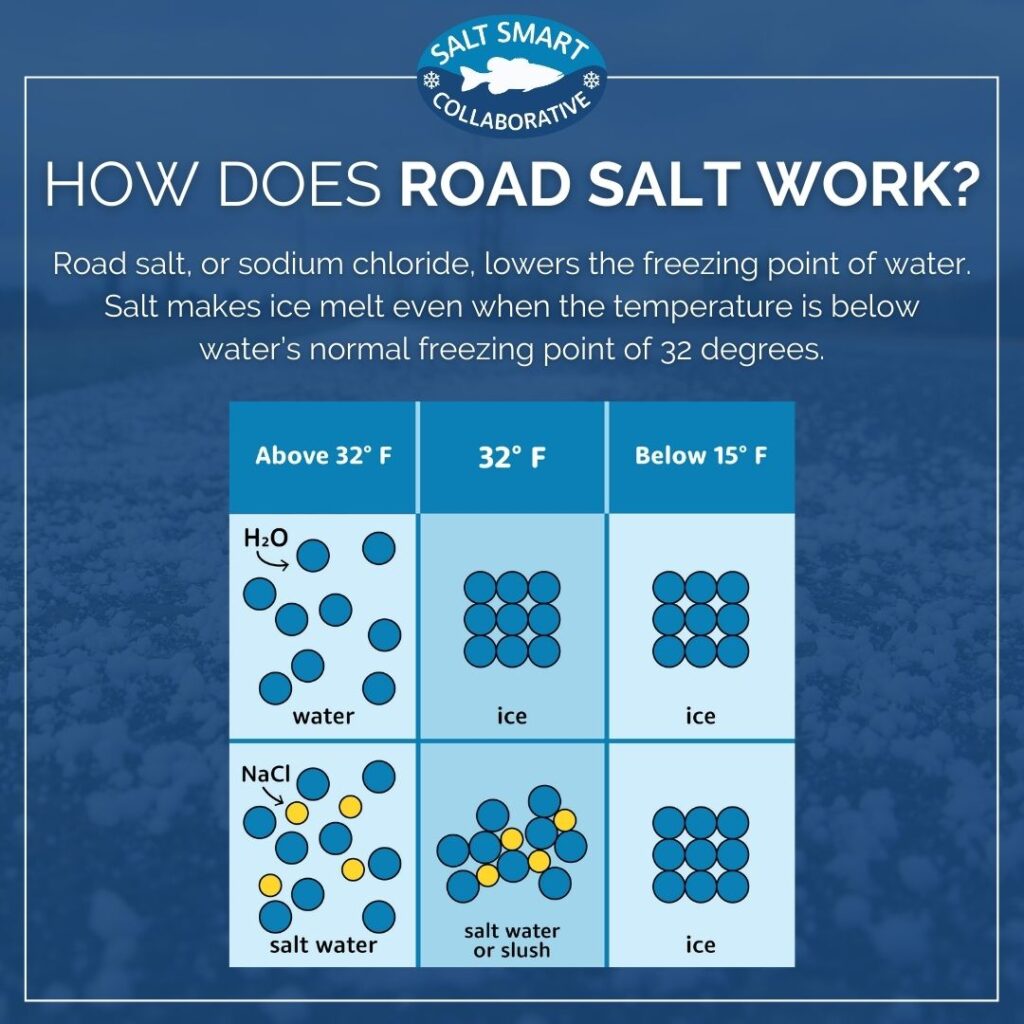
from saltsmart.org
why does salt melt ice. it is well known that when you add salt to ice, the ice not only melts but will actually get colder. when salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same. This phenomenon is called freezing point depression. Salt lowers the temperature of ice water. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. When you mix salt onto that layer, it slowly lowers its melting point. The melting process is at the. The salt has to dissolve into its ions in order to work. So, more ice melts than forms.
How Does Salt Melt Snow and Ice? Salt Smart Collaborative
Why Does Salt Slow Down Melting Of Ice The melting process is at the. it is well known that when you add salt to ice, the ice not only melts but will actually get colder. Salt lowers the temperature of ice water. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. why does salt melt ice. The melting process is at the. The salt has to dissolve into its ions in order to work. So, more ice melts than forms. salt makes ice water colder by lowering the temperature at which water freezes. when salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same. Melting ice absorbs energy, lowering the temperature. When you mix salt onto that layer, it slowly lowers its melting point. The more surface area salt can cover, the better the chances for melting ice. Salt only helps if there is a little bit of liquid water available. This phenomenon is called freezing point depression.
From id.hutomosungkar.com
Why Does Ice Melt Salt Hutomo Why Does Salt Slow Down Melting Of Ice Melting ice absorbs energy, lowering the temperature. When you mix salt onto that layer, it slowly lowers its melting point. it is well known that when you add salt to ice, the ice not only melts but will actually get colder. salt makes ice water colder by lowering the temperature at which water freezes. This phenomenon is called. Why Does Salt Slow Down Melting Of Ice.
From huntingwaterfalls.com
Why Does Salt Melt Ice? (Simple Science Explained) Why Does Salt Slow Down Melting Of Ice Salt lowers the temperature of ice water. This phenomenon is called freezing point depression. Salt only helps if there is a little bit of liquid water available. The melting process is at the. when salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same. The salt has to dissolve into. Why Does Salt Slow Down Melting Of Ice.
From pagingfunmums.com
Melting Ice & Salt Science Experiment Why Does Salt Slow Down Melting Of Ice So, more ice melts than forms. This phenomenon is called freezing point depression. The salt has to dissolve into its ions in order to work. salt makes ice water colder by lowering the temperature at which water freezes. The more surface area salt can cover, the better the chances for melting ice. Salt only helps if there is a. Why Does Salt Slow Down Melting Of Ice.
From www.wusa9.com
Why does salt make ice melt? Explaining freezing point depression Why Does Salt Slow Down Melting Of Ice salt makes ice water colder by lowering the temperature at which water freezes. when salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same. Melting ice absorbs energy, lowering the temperature. why does salt melt ice. The salt has to dissolve into its ions in order to work.. Why Does Salt Slow Down Melting Of Ice.
From saltsmart.org
How Does Salt Melt Snow and Ice? Salt Smart Collaborative Why Does Salt Slow Down Melting Of Ice salt makes ice water colder by lowering the temperature at which water freezes. Salt only helps if there is a little bit of liquid water available. it is well known that when you add salt to ice, the ice not only melts but will actually get colder. When you mix salt onto that layer, it slowly lowers its. Why Does Salt Slow Down Melting Of Ice.
From huntingwaterfalls.com
Why Does Salt Make Ice Colder? THE ACTUAL REASON Why Does Salt Slow Down Melting Of Ice Melting ice absorbs energy, lowering the temperature. The more surface area salt can cover, the better the chances for melting ice. Salt only helps if there is a little bit of liquid water available. when salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same. The salt has to dissolve. Why Does Salt Slow Down Melting Of Ice.
From www.worldatlas.com
Why Does Salt Melt Ice? WorldAtlas Why Does Salt Slow Down Melting Of Ice The melting process is at the. it is well known that when you add salt to ice, the ice not only melts but will actually get colder. Salt lowers the temperature of ice water. This phenomenon is called freezing point depression. So, more ice melts than forms. Melting ice absorbs energy, lowering the temperature. salt makes ice water. Why Does Salt Slow Down Melting Of Ice.
From www.metoffice.gov.uk
Sea ice in the climate system Met Office Why Does Salt Slow Down Melting Of Ice Salt only helps if there is a little bit of liquid water available. Salt lowers the temperature of ice water. So, more ice melts than forms. This phenomenon is called freezing point depression. The salt has to dissolve into its ions in order to work. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. . Why Does Salt Slow Down Melting Of Ice.
From www.thoughtco.com
Why Does Salt Melt Ice? Understanding How It Works Why Does Salt Slow Down Melting Of Ice This phenomenon is called freezing point depression. The melting process is at the. The salt has to dissolve into its ions in order to work. Melting ice absorbs energy, lowering the temperature. salt makes ice water colder by lowering the temperature at which water freezes. it is well known that when you add salt to ice, the ice. Why Does Salt Slow Down Melting Of Ice.
From way2estudy.blogspot.com
eduworld Why Does Salt Melt Ice? Why Does Salt Slow Down Melting Of Ice Melting ice absorbs energy, lowering the temperature. When you mix salt onto that layer, it slowly lowers its melting point. Salt lowers the temperature of ice water. The more surface area salt can cover, the better the chances for melting ice. Salt only helps if there is a little bit of liquid water available. So, more ice melts than forms.. Why Does Salt Slow Down Melting Of Ice.
From huntingwaterfalls.com
Why Does Salt Melt Ice? (Simple Science Explained) Why Does Salt Slow Down Melting Of Ice This phenomenon is called freezing point depression. it is well known that when you add salt to ice, the ice not only melts but will actually get colder. salt makes ice water colder by lowering the temperature at which water freezes. Salt only helps if there is a little bit of liquid water available. The salt has to. Why Does Salt Slow Down Melting Of Ice.
From www.youtube.com
Why does salt melt ice on roads? YouTube Why Does Salt Slow Down Melting Of Ice When you mix salt onto that layer, it slowly lowers its melting point. why does salt melt ice. Melting ice absorbs energy, lowering the temperature. Salt only helps if there is a little bit of liquid water available. Salt lowers the temperature of ice water. So, more ice melts than forms. This phenomenon is called freezing point depression. The. Why Does Salt Slow Down Melting Of Ice.
From medium.com
The Science Behind Why Salt Melts Ice by Gaia Enterprises Medium Why Does Salt Slow Down Melting Of Ice You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. Salt only helps if there is a little bit of liquid water available. it is well known that when you add salt to ice, the ice not only melts but will actually get colder. salt makes ice water colder by lowering the temperature at. Why Does Salt Slow Down Melting Of Ice.
From www.branchor.com
Why Does Salt Melt Ice? Understanding the Science and Benefits of Using Why Does Salt Slow Down Melting Of Ice The salt has to dissolve into its ions in order to work. why does salt melt ice. When you mix salt onto that layer, it slowly lowers its melting point. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. salt makes ice water colder by lowering the temperature at which water freezes. The. Why Does Salt Slow Down Melting Of Ice.
From mommaowlslab.blogspot.com
Momma Owl's Lab Melting Ice with Salt Why Does Salt Slow Down Melting Of Ice So, more ice melts than forms. salt makes ice water colder by lowering the temperature at which water freezes. Melting ice absorbs energy, lowering the temperature. why does salt melt ice. The salt has to dissolve into its ions in order to work. The more surface area salt can cover, the better the chances for melting ice. This. Why Does Salt Slow Down Melting Of Ice.
From safepaw.com
The Science Behind Why Salt Melts Ice An InDepth Explanation Why Does Salt Slow Down Melting Of Ice The salt has to dissolve into its ions in order to work. This phenomenon is called freezing point depression. The melting process is at the. When you mix salt onto that layer, it slowly lowers its melting point. Salt lowers the temperature of ice water. it is well known that when you add salt to ice, the ice not. Why Does Salt Slow Down Melting Of Ice.
From www.crewtracker.com
Why Does Salt Melt Ice? Why Does Salt Slow Down Melting Of Ice The melting process is at the. Melting ice absorbs energy, lowering the temperature. Salt lowers the temperature of ice water. it is well known that when you add salt to ice, the ice not only melts but will actually get colder. The more surface area salt can cover, the better the chances for melting ice. salt makes ice. Why Does Salt Slow Down Melting Of Ice.
From trangtraigarung.com
Why Does Ice Melt Slower In Salt Water? Exploring The Science Why Does Salt Slow Down Melting Of Ice The more surface area salt can cover, the better the chances for melting ice. The melting process is at the. The salt has to dissolve into its ions in order to work. This phenomenon is called freezing point depression. So, more ice melts than forms. Melting ice absorbs energy, lowering the temperature. salt makes ice water colder by lowering. Why Does Salt Slow Down Melting Of Ice.